-
CHUCKERSPARADISE NEWS: Click HERE to read.
QB132 V2 Quantum Boards × 1 V2 3000K
- Thread starter TerpyTyrone
- Start date
TerpyTyrone
LED Recruiter
Erick31876
Solo cup champion 2019
I have that same exact bowl or pot . Whatever you call it.
shimz
vapest
I'm sorry, but I can't let this slide. In the scenario you describe, since the input power remains the same, the heat output would remain exactly the same. Yes, you could relocate the driver to remove that portion of the heat load.You can get the lights to run cooler by increasing the area over which the power is applied (ie, same wattage, but more chips or more boards, = cooler temps). Although you can't make the driver run cooler, so if you keep the drivers outside the tent you'll manage inside ambient easier.
TerpyTyrone
LED Recruiter
He means having more wattage than I need and turning em down.I'm sorry, but I can't let this slide. In the scenario you describe, since the input power remains the same, the heat output would remain exactly the same. Yes, you could relocate the driver to remove that portion of the heat load.
IrocZ
Really Active Member
I firmly disagree.I'm sorry, but I can't let this slide. In the scenario you describe, since the input power remains the same, the heat output would remain exactly the same. Yes, you could relocate the driver to remove that portion of the heat load.
The heat output will go down when adding more boards to the same driver. If input power is 240 watts with 4 boards @60 watts each, running 8 boards @30 watts each(same 240 watts) will run cooler.
TerpyTyrone
LED Recruiter
Dude he was saying more boards and more drivers duh.I firmly disagree.
The heat output will go down when adding more boards to the same driver. If input power is 240 watts with 4 boards @60 watts each, running 8 boards @30 watts each(same 240 watts) will run cooler.
TerpyTyrone
LED Recruiter
Ok mine is 75 watts MAX! They obviously dont get run at that shit thw recommwnded driver is only,240 watts. From hlg website.
He is,saying run another setup and have the driver turned down. An led creates mlre heat thw more watts going through.
My qb132 can handle 75 but when,I,had my 240 watt driver turned all thw way up,u bet ur ass they git hotter. I,have em,down to 75% or around 200 w per light and the ambient Temp of the room is a perfect 75.
If I,wanted to get up to the recommended 600 watts for my tent then I can add an identical setup and the watts would,be 600 with all three lights turned down. But i want a qb288 r spec. They have a max rating of
VDC Current (mA) Watts at Board Lm/W at Board (55C) μmoles/joule at Board (55C)
45.72 500 22.86 206 3.00
46.98 1050 49.33 196.10 2.85
47.70 1400 66.78 190.50 2.77
48.96 2100 102.82 180.77 2.63
49.50 2400 118.80 177.50 2.58
49.86 2800 139.60 173.80 2.53
Wowwwww 140 watts!!!
Shit man
He is,saying run another setup and have the driver turned down. An led creates mlre heat thw more watts going through.
My qb132 can handle 75 but when,I,had my 240 watt driver turned all thw way up,u bet ur ass they git hotter. I,have em,down to 75% or around 200 w per light and the ambient Temp of the room is a perfect 75.
If I,wanted to get up to the recommended 600 watts for my tent then I can add an identical setup and the watts would,be 600 with all three lights turned down. But i want a qb288 r spec. They have a max rating of
VDC Current (mA) Watts at Board Lm/W at Board (55C) μmoles/joule at Board (55C)
45.72 500 22.86 206 3.00
46.98 1050 49.33 196.10 2.85
47.70 1400 66.78 190.50 2.77
48.96 2100 102.82 180.77 2.63
49.50 2400 118.80 177.50 2.58
49.86 2800 139.60 173.80 2.53
Wowwwww 140 watts!!!
Shit man
IrocZ
Really Active Member
You can get the lights to run cooler by increasing the area over which the power is applied (ie, same wattage, but more chips or more boards, = cooler temps). Although you can't make the driver run cooler, so if you keep the drivers outside the tent you'll manage inside ambient easier.
TerpyTyrone
LED Recruiter
Maybe someone else can dumb it down,for,ya
SaintyMcCunt
The Convict
Then when it's dumbed down for you, can you dumb it to the extreme for me.. please.
Just trying to prevent the spread of misinformation, but sure maybe someone could dumb it down for me. lol
This led shit makes no sense to me, to many numbers and letters and percentages
ChiefRunningPhist
Drunk on Knowledge
Couple things. Firstly, I think you're talking about the total energy of the system. I'd agree with you in that sense. The energy doesn't go away, it's still conserved. Secondly, the grow room is not a closed system.I'm sorry, but I can't let this slide. In the scenario you describe, since the input power remains the same, the heat output would remain exactly the same. Yes, you could relocate the driver to remove that portion of the heat load.
When you double your QBs from 8 to 4 but still run 600w on the driver, the area that the 600w is now being dissipated by is doubled. The thermal energy or heat per QB is halved. Also because of the reduced heat and per chip current flow, more light is produced per watt than before. So while maintaining the 1st law of thermodynamics we see that the intial thermal produced is less than previously realized and because the tent is not an absolute barrier (light energy is convected out) reducing the amount of initial thermal produced can make it locally cooler than previously, even though the total energy of the system is conserved.
Heat is conducted to the heatsink and then convected to the air. The rate at which the thermal energy is transferred depends on the size of the gradient.
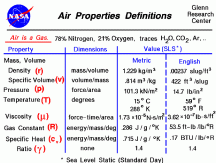
The density of air is small. At 1.225kg/m3 a 4x4x7ft tent has ~3.85kg of air, a 10x10x8 room has ~27.75kg of air. 600w equals to 600J/s. Air has a Cp (specific heat capacity) of ~1. So to move the tent air 1°C we need 3.85kJ of energy, and to move the room air 1°C we need 27.75kJ of energy. In 1 min 600w produces 36kJ of energy.
Obviously there's more in the system than just air. The heatsinks absorb energy, the tent absorbs energy, the plants, the pots, everything, grow room walls, everything, they all conduct thermal throughout themselves and convect to the outside as well. You can see at 36kJ/min the tent should be on fire within the hr lol (36kJ × 60min = 2,160kJ) ... So its really hard to apply theoretical thermo without knowing all the variables, Cp's and thermal conductivities masses, ect. Though judging based off of initial thermal production seems to have the most consistent correlation to ambient and is supported by the real world measurements of cooler temps with less concentrated power. The rate at which the energy is transferred into thermal and the rate at which the system conducts thermal away matters.

EDIT:
3.79kg/gal water
0.00379m3/gallon of water
Cp of water = 76 kJ/kg·K°
~288kJ to move 0.00379m3 of water 1°C
~27kJ to move ~22m3 of air
~76,000.00kJ to raise 1m3 of water 1°C
~1.22kJ to raise 1m3 of air 1°C
A small change in energy being added to 1m3 of air is much more noticeable than a small change in energy being added to 1m3 of water. The less energy being convected or transferred to the air intially the lower the ambient temp. If light is shining on walls the air is not absorbing the light energy. Air absorbs visible light poorly.
Last edited:
TerpyTyrone
LED Recruiter
Yahoooi!!!!Couple things. Firstly, I think you're talking about the total energy of the system. I'd agree with you in that sense. The energy doesn't go away, it's still conserved. Secondly, the grow room is not a closed system.
When you double your QBs from 8 to 4 but still run 600w on the driver, the area that the 600w is now being dissipated by is doubled. The thermal energy or heat per QB is halved. Also because of the reduced heat and per chip current flow, more light is produced per watt than before. So while maintaining the 1st law of thermodynamics we see that the intial thermal produced is less than previously realized and because the tent is not an absolute barrier (light energy is convected out) reducing the amount of initial thermal produced can make it locally cooler than previously, even though the total energy of the system is conserved.
Heat is conducted to the heatsink and then convected to the air. The rate at which the thermal energy is transferred depends on the size of the gradient.
The density of air is small. At 1.225kg/m3 a 4x4x7ft tent has ~3.85kg of air, a 10x10x8 room has ~27.75kg of air. 600w equals to 600J/s. Air has a Cp (specific heat capacity) of ~1. So to move the tent air 1°C we need 3.85kJ of energy, and to move the room air 1°C we need 27.75kJ of energy. In 1 min 600w produces 36kJ of energy.
Obviously there's more in the system than just air. The heatsinks absorb energy, the tent absorbs energy, the plants, the pots, everything, grow room walls, everything, they all conduct thermal throughout themselves and convect to the outside as well. You can see at 36kJ/min the tent should be on fire within the hr lol (36kJ × 60min = 2,160kJ) ... So its really hard to apply theoretical thermo without knowing all the variables, Cp's and thermal conductivities masses, ect. Though judging based off of initial thermal production seems to have the most consistent correlation to ambient and is supported by the real world measurements of cooler temps with less concentrated power. The rate at which the energy is transferred into thermal and the rate at which the system conducts thermal away matters.
View attachment 7031
View attachment 7032
EDIT:
3.79kg/gal water
0.00379m3/gallon of water
Cp of water = 76 kJ/kg·K°
~288kJ to move 0.00379m3 of water 1°C
~27kJ to move ~22m3 of air
~76,000.00kJ to raise 1m3 of water 1°C
~1.22kJ to raise 1m3 of air 1°C
A small change in energy being added to 1m3 of air is much more noticeable than a small change in energy being added to 1m3 of water. The less energy being convected or transferred to the air intially the lower the ambient temp. If light is shining on walls the air is not absorbing the light energy. Air absorbs visible light poorly.
View attachment 7036
I knew someone could do it!!
I was trying to feed my 4 month old and make mama happy, but I couldnt fight the urge to try and explain it. Thanks @chief you have a way with words!
TerpyTyrone
LED Recruiter
Thats why my basement acted like a heat sink from mar til June when the ground thawed. Until then i coyld keep the ambient temp of the concrete walls nice and cool with a 1000w hps anx air movement.
Its not as easy to cool air in a tent.
If you have 10 40 watt cfls dimmed @ 50% in a room they will be less warm than 5 40watt cfls @ 100%
Its not as easy to cool air in a tent.
If you have 10 40 watt cfls dimmed @ 50% in a room they will be less warm than 5 40watt cfls @ 100%
Erick31876
Solo cup champion 2019
When I hear people talking about that LED stuff it's like they're speaking Chinese to meThen when it's dumbed down for you, can you dumb it to the extreme for me.. please.
This led shit makes no sense to me, to many numbers and letters and percentages
shimz
vapest
Lol, love it. Thanks for the analysis, but since the open system remains the same (the tent and it's contents), my argument stands. You will heat the surroundings at the same rate regardless of number of diodes in the system. Yes, you gain some photon efficiency, but guess what? Those photons' energy become heat added to the system. You're on the right track with your comment on thermodynamics. Keep studying.Couple things. Firstly, I think you're talking about the total energy of the system. I'd agree with you in that sense. The energy doesn't go away, it's still conserved. Secondly, the grow room is not a closed system.
When you double your QBs from 8 to 4 but still run 600w on the driver, the area that the 600w is now being dissipated by is doubled. The thermal energy or heat per QB is halved. Also because of the reduced heat and per chip current flow, more light is produced per watt than before. So while maintaining the 1st law of thermodynamics we see that the intial thermal produced is less than previously realized and because the tent is not an absolute barrier (light energy is convected out) reducing the amount of initial thermal produced can make it locally cooler than previously, even though the total energy of the system is conserved.
Heat is conducted to the heatsink and then convected to the air. The rate at which the thermal energy is transferred depends on the size of the gradient.
The density of air is small. At 1.225kg/m3 a 4x4x7ft tent has ~3.85kg of air, a 10x10x8 room has ~27.75kg of air. 600w equals to 600J/s. Air has a Cp (specific heat capacity) of ~1. So to move the tent air 1°C we need 3.85kJ of energy, and to move the room air 1°C we need 27.75kJ of energy. In 1 min 600w produces 36kJ of energy.
Obviously there's more in the system than just air. The heatsinks absorb energy, the tent absorbs energy, the plants, the pots, everything, grow room walls, everything, they all conduct thermal throughout themselves and convect to the outside as well. You can see at 36kJ/min the tent should be on fire within the hr lol (36kJ × 60min = 2,160kJ) ... So its really hard to apply theoretical thermo without knowing all the variables, Cp's and thermal conductivities masses, ect. Though judging based off of initial thermal production seems to have the most consistent correlation to ambient and is supported by the real world measurements of cooler temps with less concentrated power. The rate at which the energy is transferred into thermal and the rate at which the system conducts thermal away matters.
View attachment 7031
View attachment 7032
EDIT:
3.79kg/gal water
0.00379m3/gallon of water
Cp of water = 76 kJ/kg·K°
~288kJ to move 0.00379m3 of water 1°C
~27kJ to move ~22m3 of air
~76,000.00kJ to raise 1m3 of water 1°C
~1.22kJ to raise 1m3 of air 1°C
A small change in energy being added to 1m3 of air is much more noticeable than a small change in energy being added to 1m3 of water. The less energy being convected or transferred to the air intially the lower the ambient temp. If light is shining on walls the air is not absorbing the light energy. Air absorbs visible light poorly.
View attachment 7036
ChiefRunningPhist
Drunk on Knowledge
Haha well you won't heat the surroundings at the same rate. I had to study this stuff and know how to apply it to be an engineer. You can pm me if you'd like.Lol, love it. Thanks for the analysis, but since the open system remains the same (the tent and it's contents), my argument stands. You will heat the surroundings at the same rate regardless of number of diodes in the system. Yes, you gain some photon efficiency, but guess what? Those photons' energy become heat added to the system. You're on the right track with your comment on thermodynamics. Keep studying.
A 100w used to charge a battery will heat the air of a 1m3 volume that it is occupying at a different rate than 100w used to power a space heater in a 1m3 area.
The total energy in will be the same, but the rate at which the energy is converted to thermal and transferred to the air won't be the same. More energy is absorbed by the surroundings vs the air in one scenario over the other, this difference is especially noticed when measuring the abient between the 2 scenarios
Last edited:








