*im not a chemical engineer and these are just thoughts and I'm not sure if I'm right and you'd want to ask actual experts to validate*
Pretty sure...
Upon further review it looks like potassium silicate will dissolve in water, but only in very small amounts at room temp and not until you get to 80C will it be ~300g/L. So boil your water to dissolve the potassium silicate. When it cools it turns into a thick honey but won't precipitate out.
Getting shaky...
Then it looks like you'd have to dissolve a lil glob of the honey you just made into some water and add some acid till there was no more bubbles till the solution was slightly acidic, and then you just made silicic acid.
Pretty sure...
But this acid needs to be stabilized with something or it will want to polymerize and form long chains and become unavailable to humans or plants, this is why they add choline or other things ect.
View attachment 84652
(From attached .pdf from previous post)
Getting shaky....
I'm not sure if its as easy as adding some choline chloride salt to your water before you add your honey glob to perform your acid titration?
Getting shaky...
You'd want to probably have more moles choline than moles of silicate.
Explaining doubt..
In the .pdf posted previously they reference dry HCl, and silicon tetrachloride, and choline chloride, so I'm not sure if an acid titration will work to create the intended molecules. The reaction might have to undergo without the presence of water.
Pretty sure...
They do then say that water is later added, and a base is introduced to neutralize the pH and then its concentrated by steam distillation under vacuum.
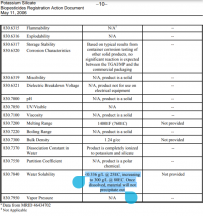
*attached is a .pdf of the chemical properties of potassium silicate (a SS of one of the tables from the .pdf is below)..
View attachment 84654
Pretty sure..
All that you're trying to do is "acidify" silicate salts (in this case potassium silicates), but then stabilize the resulting silicic acid that you got from "acidifying" the silicate salts with something like choline or vanillin ect so that the "acidified" silicates stay small and in bio available form.