Light is made up of things called photons. They exhibit wave particle duality which means they act like both a particle and a wave.
A red laser light is made up of red photons all flying through the air pointed in the same direction. A blue laser light is made up of blue photons all flying through the air pointed in the same direction. A green LED light is made up of green photons all flying through the air pointed in all sorts of directions. An orange LED light is made up of orange photons flying through the air pointed in all sorts of directions. Wherever there is light, its because photons are present.
A "mole" is like the term million or billion, its just a big unit-less number (it isn't used for only ft, or only miles, or only dollar bills, it can be used to describe anything, so it's unit-less), you can have 10 million, 20 million, 33 million, ect, you can also have 10 moles or 20 moles or 33 moles, ect.
1 mole = 602,214,076,000,000,000,000,000
or...
1 mole = 6.022e23
The symbol "μ" means micro, or means that any unit label that it is attached to is divided by 1,000,000 (million). So a micro-mole, or a μmol, is the amount of a mole that's been divided by a million...
μ = micro
μ = (1/1,000,000)
μ = 0.000001
μ = 1e-6
μ = 1 × 10^(-6)
^^^All means the same thing
μmol = (0.000001) × (602,214,076,000,000,000,000,000)
μmol = 602,214,076,000,000,000
vvv means the same thing ^^^
μmol = (1e-6) × (6.022e23)
μmol = 6.022e17
2μmol's of photons is 2 × (6.022e17) photons. That's a mouthful and when you have so many particles it's easier to just say 2μmol's or 3μmol's, same with 1 million, we don't say 10 one-hundred thousands, we say 1 million, same thing, it's just an amount of something. A mole of weed would NOT be correct terminology because it would just be like saying a million weed lol. A million what weed? A million grams of weed? A million oz of weed, a million what? Same thing with moles they are unit-less, so you'd describe it as moles of grams of weed, or moles of ounces of weed, or X amount of moles of something.
Photons are pretty much just pieces of energy themselves. They do act like particles as they have momentum, but they are essentially an intermediary where energy is stored until the photon is absorbed and dissipates its energy into the absorbing molecule.
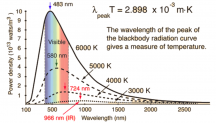
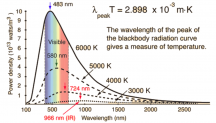
Photons have/are energy, but the amount of energy is not the same for every photon. Some photons have more energy (UV end) and some have less energy (IR end). White light is a mix of all sorts of different energy photons between UV and IR. Bunches of red photons, and orange photons, and yellow photons, and green photons, ect. Your eyes and brain is the one that blends them and tricks you into seeing white, but really they are just a big ol mix of lots of different energy photons, or different color photons being emitted at once. When you mix red and blue light you get purple, but not really, it's just your eyes tricking you, the red and blue photons didn't morph into purple photons, they stayed the same.
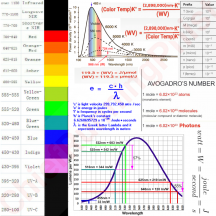
Another way of describing the different energies of the photons is by wavelength. The longer the wavelength, the lower the energy of the photon, the shorter the WV the higher the energy of the photon.
View attachment 9895
A blue photon or a short wavelength photon has about 1.5x more energy in itself than a red photon or a long wavelength photon.
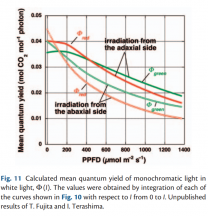
The reason we care about photons is because that's how photosynthesis takes place. The plant has to absorb about 9 moles of photons to evolve 1 mole of CO2 into 1 mole of O2 and 1/6 mole of glucose.
View attachment 9896
That's what it does all day long, absorbs 9 photons, boom, converts it to glucose, absorbs 9 photons, boom, coverts it to glucose (really somewhere between 8-10 photons). Photosynthesis doesn't care so much about the type or the energy of the photons absorbed, just the total number.
Photons can have different energies, but photosythensis only cares about the total number absorbed. This means that we can use any photons that are absorbed (regardless their wavelength or energy) to drive photosynthesis. So the difference between 9 IR photons (low energy) driving photosynthesis and 9 UV photons (high energy) driving photosynthesis is the total energy that each group of photons adds up to. The plant got 9 photons in each example, but the amount of energy that it received was different.
Lets say the 9 IR photons were 0.15 joules of energy per each photon, and lets say the 9 UV photons were 0.30 joules of energy per each photon.
9 × 0.15 = 1.35 joules in 9 photons of IR
and...
9 × 0.30 = 2.7 joules in 9 photons of UV.
Each amount of energy was still 9 photons so photosynthesis was happy, but you can see that the IR was more effecient, or it only took 1.35J of energy to make 9 photons to then drive photosynthesis, whereas the UV took 2.7J of energy to make 9 photons to then drive photosynthesis.
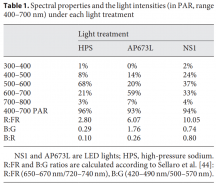
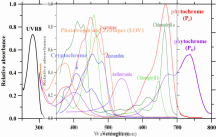
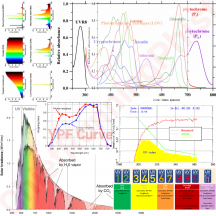
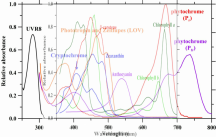
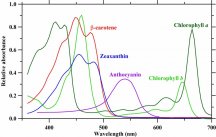
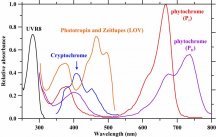
The plant absorbs certain energy photons better than others, or certain wavelengths better than others. So where it might only require 9 photons to drive photosynthesis, the plant may get those 9 photons easier with 1 wavelength over another wavelength. Absorption curves show what energy the photons are absorbed at the best, or what wavenlegth the photons are absorbed at the best. Each one of these lines is a different plant molecule that absorbs light for the plant, they have different absorption ranges...
View attachment 9891
If a WV is absorbed poorly, like green (reflected, thus why plants are green), then it will take lots longer to get 9 photons compared to red which is readily absorbed. Maybe 1 out of every 10 green photons gets absorbed but 8 out of every 10 red photons gets absorbed, you'd have to wait longer for the plant to absorb 9 green photons vs 9 red photons, or you'd have to use a ton of green photons compared to red photons in order for the plant to satisfy its absorption of 9 photons.
PPFD is just the amount of photons that are bombarding a square meter each second. They use the μmol term though. 1100PPFD means 1,100μmol's of photons per m2 every second. We already found out just how big a mole really is, so it's really easier not to try to think of PPFD as 1100 trillion triilion billion photons (or whatever it is), but rather just start getting comfortable with gauging μmol as a new measurement that your brain will acclimate to over time. Just like trying to think of what a kilometer meant before you really knew, sure, you knew it was 1000m, but what did that really mean(?), over time you grew to get a feel for what a km meant.
WV ÷ 119.3 = μmol/J
119.3 ÷ WV = J/μmol
If you want to know the energy of a wavelength (WV) then divide 119.3 by the WV and you'll calculate how much energy is in 1μmol of photons in that WV. If you want to know how many photons of a certain WV can be created with 1 joule of energy, then divide WV by 119.3 and you'll calculate the number of photons of that WV per joule of energy.
When people talk about a fixture being 2.3μmol/J, what that means is that the light is emmiting 2.3μmol's of photons for every J it consumes. What is a joule? A joule is a unit of energy and (Watts = J/s) watts equal joules per second. So if the light consumes energy in watts, lets say 100W (or 100J/s), then subsequently every second the light consumes 100J, and if it emits 2.3μmol/J, then 100J/s × 2.3μmol/J= 230μmol/s. If this amount of light falls in 1m2 area, then that m2 would have a PPFD of 230μmol/s per m2. Lets say you had 500W of 2.3μmol/J effeciency, then 500J/s × 2.3μmol/J = 1,150μmol/s, and if this amount of photons fell on 1m2 then it would have a PPFD of 1,150μmol/s per m2.
PPFD only tells you the number of photons that are present on an average basis over a m2. It doesn't tell you which photons, which wavelengths or energies. If we recall back to the phenomenon of the plant absorbing different photons better than others, we can see that PPFD can give an idea but just having a high PPFD doesn't mean the plant will absorb every photon the same. Now, if the PPFD were high and it were made up of the photons that were easily absorbed then it's going to be a significant metric, but if the PPFD is high but in a wavelength that isn't absorbed well, then its going to perform poorly despite the high PPFD, so the PPFD in this instance would give a false indicator on how well it could grow a plant.
EDIT:
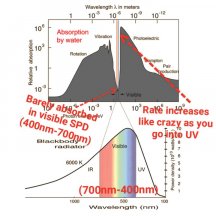
PAR - photosynthetically active radiation; light between 400nm - 700nm
PPFD - photosynthestic photon flux density; light between 400nm - 700nm, measured in μmol/s per m2
PFD - photon flux density; light between 380nm - 780nm, measured in μmol/s per m2